
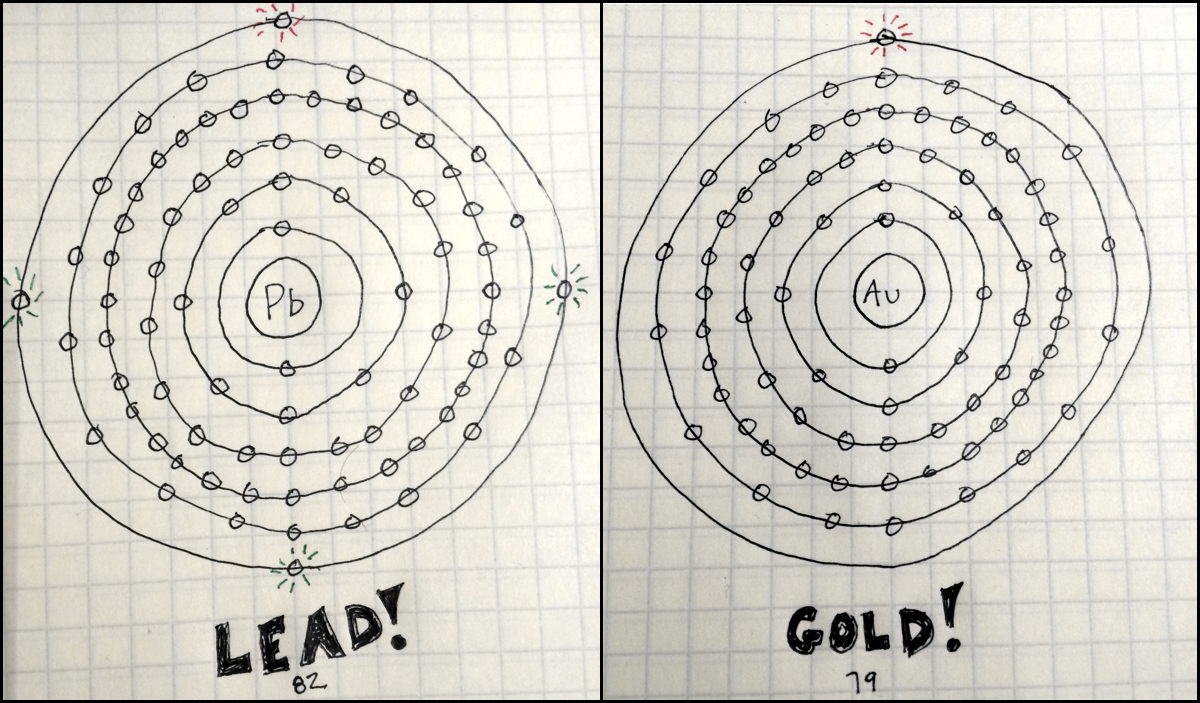
It must be noted, atoms lack a well-defined outer boundary. The atomic radius of Lead atom is 146pm (covalent radius). Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Mass numbers of typical isotopes of Lead are 204-208. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.įor stable elements, there is usually a variety of stable isotopes.

Neutron number plus atomic number equals atomic mass number: N+Z=A. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. "The activated tissue can also remove glucose from the blood, which can help control diabetes," he added.Atomic Number – Protons, Electrons and Neutrons in Lead "Our work shows how a regulator binds to prevent UCP1 activity, but more importantly the structure will allow scientists to rationalise how activating molecules bind to switch the protein on, leading to the burning of fat," said lead researcher Edmund Kunji, from the University of Cambridge.

"This is an exciting development that follows more than four decades of research into what UCP1 looks like and how it works," said Vera Moiseenkova-Bell, an associate professor at the Beckman Center for Cryo-Electron Microscopy, US. Using the Krios G3i, a cryogenic electron microscope at the Singh Center for Nanotechnology at the University of Pennsylvania, US, the team was able to view UCP1 in atomic detail. The new study shows, for the first time, the structure of UCP1 in atomic detail, and how its activity in brown fat cells is inhibited by a key regulatory molecule, the researchers said.
Despite more than 40 years of research, we did not know what UCP1 looks like to understand how it worksuntil now," Crichton said. And research has been hampered by a lack of details on the molecular make up of UCP1.
#Lead atomic structure full
"But even with more brown fatUCP1 must still be 'switched on' to gain full benefit. "UCP1 is the key protein that allows the specialised brown fat to burn off calories as heat," said Paul Crichton from the the University of East Anglia (UEA), UK. Most of our fat, however, is white fat, which stores energy, and too much white fat leads to obesity.
The finding, published in the journal Science Advances, could one day combat obesity and related diseases, such as diabetes, they said.īrown fat is the good fat - it breaks down blood sugar and fat molecules to create heat and help maintain body temperature. The protein called "Uncoupling protein 1" (UCP1) allows brown fat tissue, or "good fat," to burn off calories as heat, in contrast to conventional white fat that stores calories.Īn international team, including researchers from the University of Cambridge, UK, provides crucial molecular details that will help develop therapeutics which activate UCP1 artificially to burn off excess calories from fat and sugar. Researchers have for the first time revealed the molecular structure of a protein that allows "good fat" to burn off calories, an advance that could lead to treatments for obesity and diabetes.


 0 kommentar(er)
0 kommentar(er)
